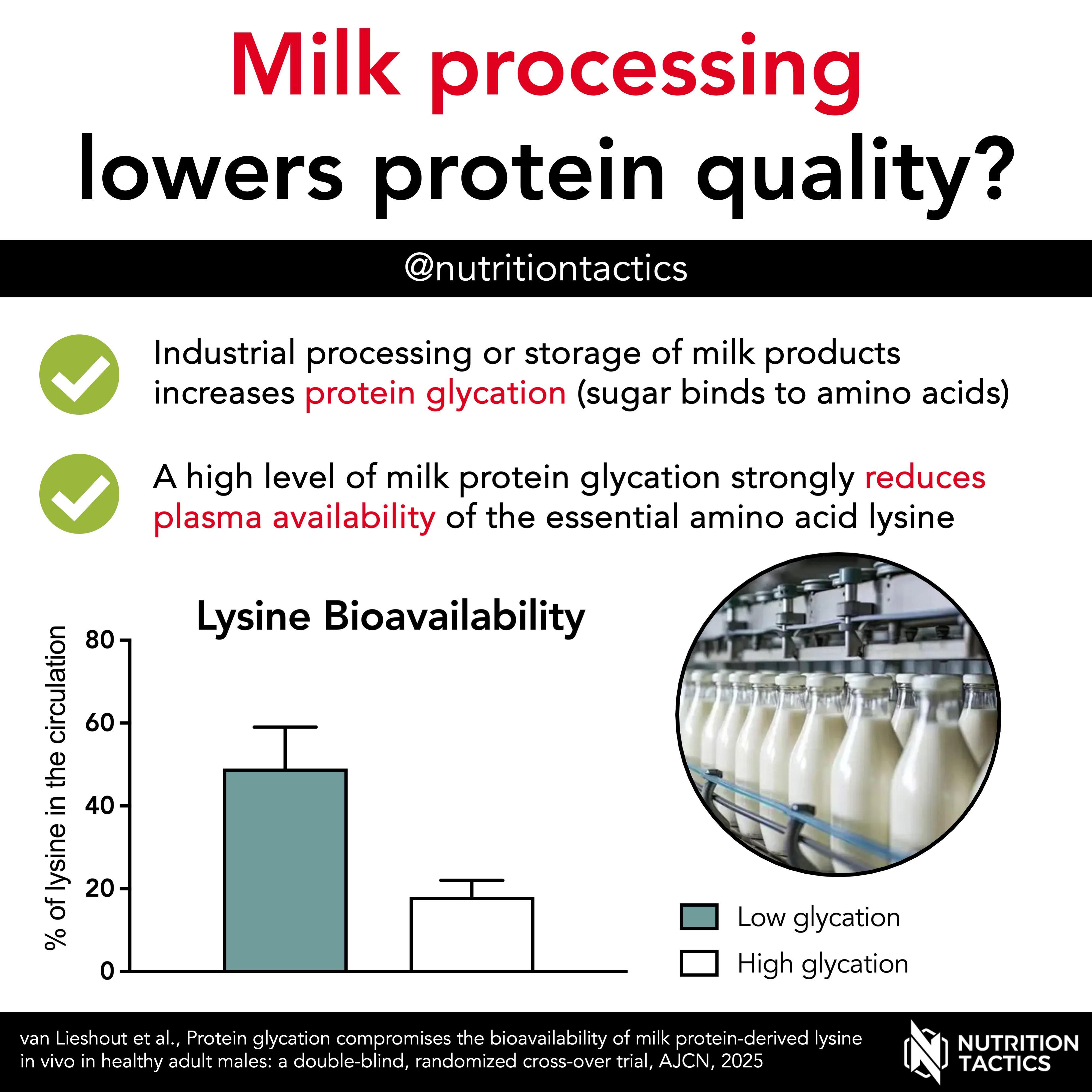
Industrial processing and storage of milk products can cause sugars to bind to amino acids, a process called protein glycation. The essential amino acid lysine is especially sensitive to this, and it’s thought that this may reduce its absorption and bioavailability.
In our new study, we compared the plasma lysine bioavailability of milk protein with a high (50%) vs low (3%) glycation level.
We used specially produced milk protein that included labeled (tracer) versions of lysine, leucine, and phenylalanine. These tracers allowed us to track how much of each amino acid from the milk protein appeared in the bloodstream after digestion and absorption.
Key finding:
- Plasma lysine bioavailability was 18% and 49% for the highly and lowly glycated protein, respectively.
- The bioavailability of leucine and phenylalanine was not affected by protein glycation.
What does this mean?
Our findings suggest that industrial processing and storage of milk protein products can substantially lower the bioavailability of lysine, thereby reducing the nutritional quality of the protein.
Unfortunately, there is no way to know how glycated specific protein products are. But if more research shows glycation can have an impact on protein quality, perhaps it will become common practice to report it on the label. Products that contain both protein and carbohydrate powder are at a higher risk than fluid products such as milk itself.
Go to the next infographic in the protein series:
Casein protein is not always slowly digested?

Leave a Reply